8.1 Metal Deposits
Steve Earle
The earliest known metal mines, in Bulgaria and Serbia and some neighbouring areas of southeastern Europe, date back about 7000 years, with the main metal of interest being copper. Copper was also mined and used in the Great Lakes region of North America at around the same time.
Today there are mines, for virtually every metal in the periodic table, on every continent except Antarctica (where mining is banned by treaty), and the value of the mined products are enormous—both to the companies that do the mining and the nations where the mining takes place, and to the people everywhere that use the metals in their lives every day. Iron represents, by far, the largest amount of metal mined. It is used mostly in construction (buildings, bridges, etc.), for railways, and for vehicles, although most manufactured products have some iron in them. Each year we mine and process 2.5 billion tonnes of iron, which is approximately the weight of all of the cars, trucks and buses currently in the entre world. Aluminum, which is used in electrical transmission lines, construction and aircraft, is second. Chromium is third because it is an important component of steel, while copper, fourth, is used widely for electrical components.
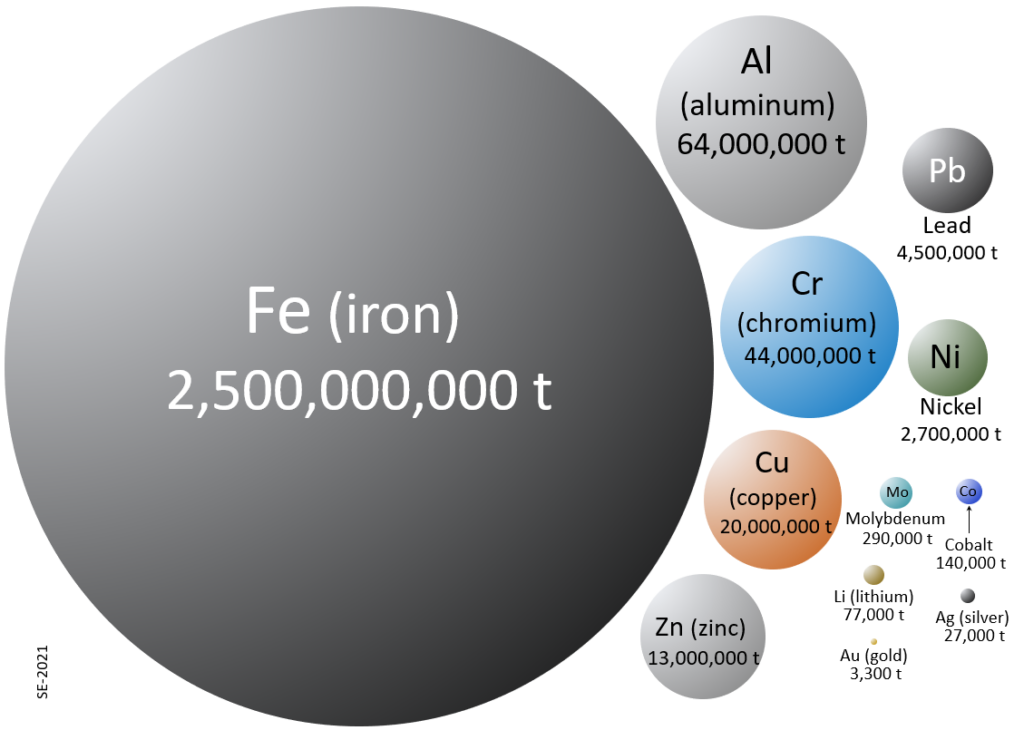
A metal deposit is a body of rock in which one or more metals has been concentrated to the point of being economically viable for recovery. Not all metal-bearing rock is useful as ore. The granitic rock of the crust has an aluminum (Al) content of about 8%, but most of that is within the mineral feldspar, and it is very difficult to separate that Al from the other elements. The main ore of Al is bauxite, in which the Al is present as Al-hydroxides. Bauxite is much more readily processed into aluminum metal than is granite.
Some background levels of important metals in average rocks are shown on Table 8.1, along with the typical grades necessary to make a viable deposit, and the corresponding concentration factors. Looking at copper, for example, we can see that while average rock has around 40 ppm (parts per million) of copper, a grade of around 10,000 ppm or 1% is necessary to make a viable copper deposit. In other words, copper ore has about 250 times as much copper as typical rock. For all of the other elements in the list the concentration factors are much higher. For gold it’s 2000 times and for silver it’s around 10,000 times.
| Metal | Typical Background Level | Typical Economic Grade | Concentration Factor |
| Copper | 40 ppm | 10,000 ppm (1%) | 250 times |
| Gold | 0.003 ppm | 6 ppm | 2000 times |
| Lead | 10 ppm | 50,000 ppm (5%) | 5000 times |
| Molybdenum | 1 ppm | 1,000 (0.1%) | 1000 times |
| Nickel | 25 ppm | 20,000 (2%) | 800 times |
| Silver | 0.1 ppm | 1,000 (0.1%) | 10,000 times |
| Uranium | 2 ppm | 10,000 (1%) | 5000 times |
| Zinc | 50 ppm | 50,000 (5%) | 1000 times |
The economic viability of any metal deposit depends on a wide range of factors including its grade, size, shape and depth below surface, proximity to infrastructure, the current price of the metal, ease of processing, the labour and environmental regulations in the area, and many others. One of the most important of these factors is the grade (the concentration of metal in the ore) and whether it is high enough to make it mineable at a profit. For example, a typical economic grade for copper is 1%, but that will vary widely depending on the status of the various factors listed above, including size, shape and depth of the deposit, ease of processing the ore, proximity to infrastructure, and the labour and environmental rules in the country where the orebody is located.[1]
From Table 8.1 we can see that some significant concentration must take place to form a mineable deposit. This concentration may occur during the formation of the host rock, or after the rock is formed, through a number of different types of processes. There is a very wide variety of ore-forming processes, and there are hundreds of types of mineral deposits. The origins and characteristics of a few of them are described below.
Magmatic Deposits
A magmatic deposit is one in which the metal concentration takes place primarily at the same time as the formation and emplacement of the magma. Most of the nickel mined in the world, and much of the copper, comes from magmatic deposits such as those in Indonesia, Canada, southern Africa, Australia and Siberia (Figure 8.1.2). The magmas from which these deposits are formed are of mafic or ultra-mafic composition (they are derived from the mantle), and therefore they have relatively high nickel and copper contents to begin with (as much as 100 times that of normal rocks in the case of nickel). These elements can be further concentrated within the magma as a result of addition of sulphur from partial melting of the surrounding rocks. The heavy nickel and copper sulphide minerals are then concentrated further still by gravity segregation (i.e., crystal settling towards the bottom of the magma chamber). In some cases, there are significant concentrations of platinum-bearing minerals in deposits of this type.
Most of magmatic deposits around the world are Precambrian in age—probably because the mantle was significantly hotter at that time, and the necessary ultramafic magmas were more likely to exist close to the surface.

Volcanogenic Massive Sulphide Deposits
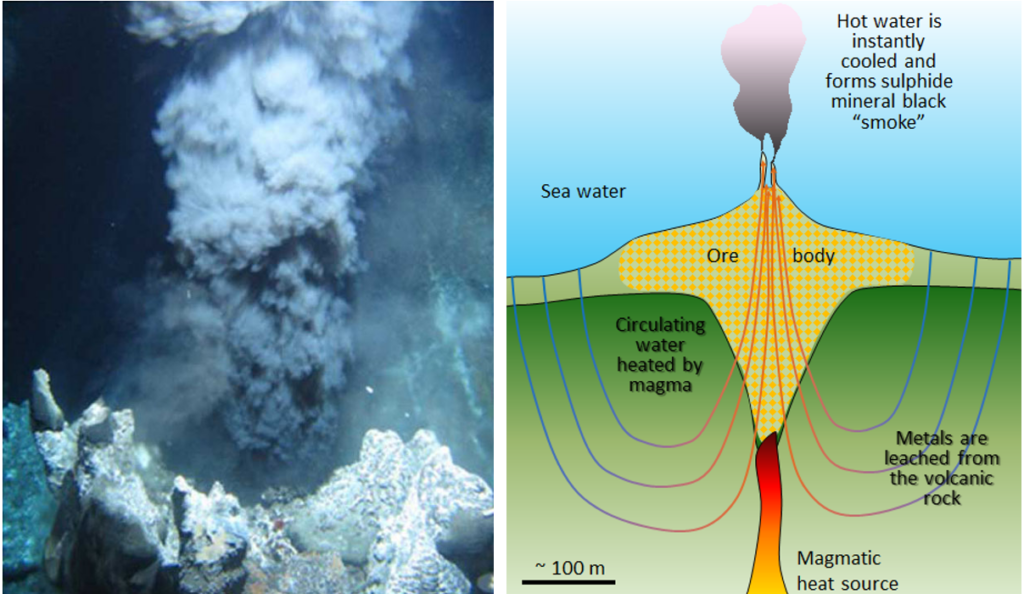
Much of the world’s copper, zinc, lead, silver and gold is mined from volcanogenic massive sulphide (VMS) deposits associated with submarine volcanism. There are similar deposits in many countries, with some of the larger ones in Spain and Portugal, in southern Africa, Canada, and Russia.[2]
VMS deposits are formed from the water discharged at high-temperature (250 to 300˚ C) at ocean-floor vents, primarily in areas of subduction-zone volcanism. Although most VMS deposits are tens to hundreds of millions of years old, the environment of their formation is comparable to that of a modern-day black smoker (Figure 8.1.3) which form where hot metal- and sulphide-rich water issues from the sea floor. They are called massive-sulphide deposits because the sulphide minerals (including pyrite (FeS2) , sphalerite (ZnS), chalcopyrite (CuFeS2) and galena (PbS)) are generally present at very high concentrations (making up the majority of the rock in some cases). The metals and the sulphur are leached out of the sea-floor rocks by convecting groundwater driven by the volcanic heat, and then quickly precipitated where that hot water cools suddenly and changes chemically on entering the cold sea water, so the process of concentration of the metal is a sudden change in metal solubility where the hot water cools quickly at the sea-floor rock-water interface. The volcanic rock that hosts VMS deposits is formed in the same area and at the same general time as the accumulation of the ore minerals.
Porphyry Deposits
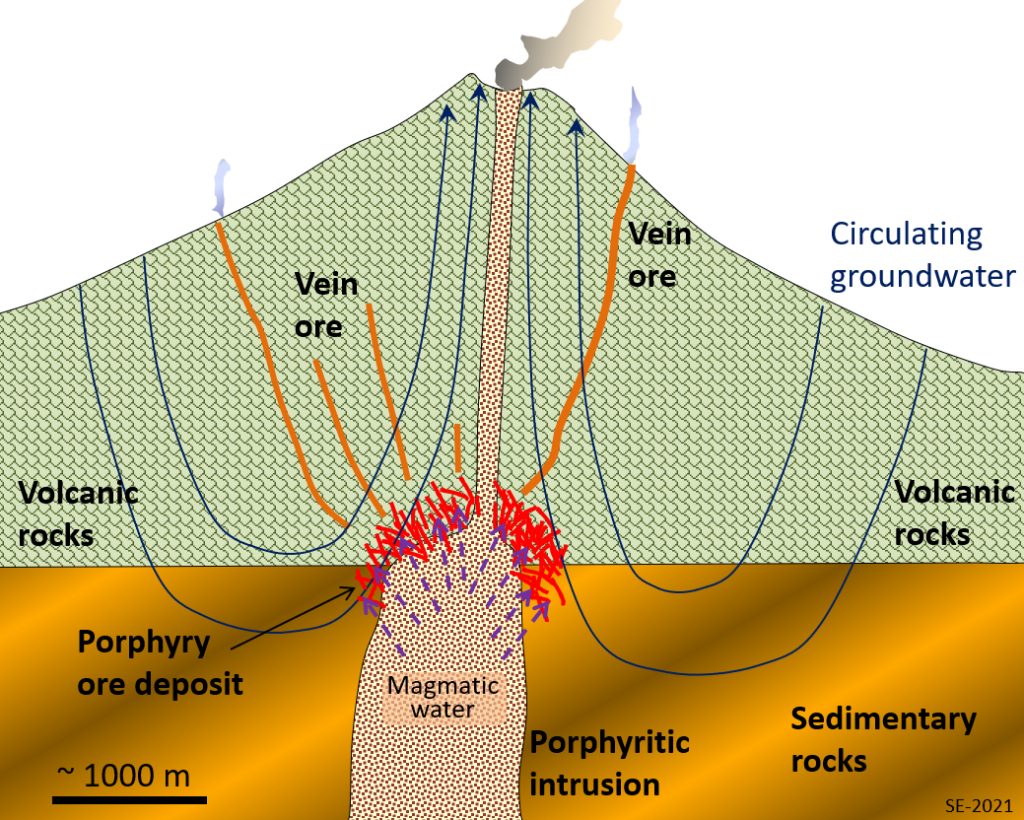
Porphyry deposits are the most important ores of copper and molybdenum in western North and South America, and in other areas of the Pacific Rim. Most porphyry deposits also include some gold, and in a few cases gold is the primary commodity.
A porphyry deposit forms around a cooling felsic stock (magma chamber) in the upper part of the crust. They are called “porphyry” because upper crustal stocks are typically porphyritic in texture, the result of a two-stage cooling process. Metal enrichment results from convection of groundwater related to the heat of the stock, and also from hot water expelled by the cooling magma (Figure 8.1.4). The host rocks, which commonly include the stock itself and the surrounding country rocks, are normally highly fractured and brecciated. During the ore-forming process some of the original minerals in these rocks get altered to potassium feldspar, biotite, epidote and various clay minerals. The important ore minerals include chalcopyrite (CuFeS2), bornite (Cu5FeS4) and pyrite in copper porphyry deposits, or molybdenite (MoS2) and pyrite in molybdenum porphyry deposits. Gold is present as minute flakes of native gold.
This type of environment (i.e., around and above an intrusive body) is also favourable for the formation of other types of deposits—particularly vein-type gold deposits (a.k.a. epithermal deposits). Many of the other gold deposits situated all along the western edge of both South and North America are of the vein type shown in Figure 8.1.4, and are related to nearby magma bodies.
Some porphyry deposits are huge, and if the ore is near to the surface they are most economically mined by open-pit methods (more on that below). The largest porphyry copper deposit in the world is at Chuquicamata in northern Chile (Figure 8.1.5). It has been in operation for over 100 years, has produced over 29 million tonnes of copper metal, and is expected to continue operation for at least another 50 years. The mine is owned by the government of Chile and supports a nearby city with a population of 160,000.

Banded Iron Formation
Most of the world’s important iron deposits are of the banded iron formation type, and most of them formed during the initial oxygenation of the Earth’s atmosphere between 2400 and 1800 Ma. At that time abundant iron in dissolved form in the ocean (as Fe2+) became oxidized to its insoluble form (Fe3+) as the Earth’s atmosphere gradually became more oxygen-rich. The iron minerals accumulated on the sea floor, mostly as hematite and magnetite interbedded with chert (Figure 8.1.6). Unlike many other metals, which are economically viable at grades of around 1% or even much less, iron deposits are only viable if the grades are in the order of 50% iron.

Unconformity-Type Uranium Deposits
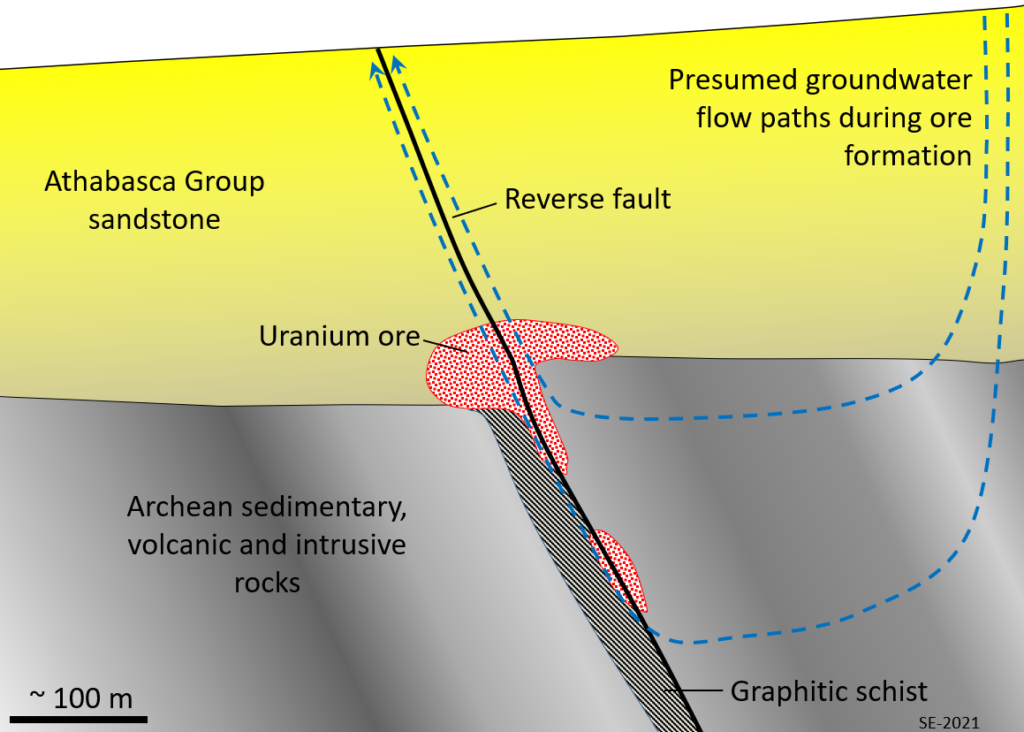
There are several different types of uranium deposits, but some of the largest and richest are those within the Athabasca Basin of northern Saskatchewan. They are called unconformity-type because they are all situated very close to the unconformity between the Proterozoic Athabasca Group sandstone and the much older Archean sedimentary, volcanic and intrusive igneous rock (Figure 8.1.7). The origin of unconformity-type U deposits is not perfectly understood, but it is thought that two particular features are important: the relative permeability of the Athabasca Group sandstone, and the presence of graphitic schist within the underlying Archean rocks. The permeability of the sandstone allowed groundwater to flow through it and leach out small amounts of U which stayed in solution in the oxidized form U6+. The graphite (C) created a reducing environment (non-oxidizing) that converted the U from U6+ to insoluble U4+, at which point it was precipitated as the mineral uraninite (UO2).
Thermal energy (heat) from within the Earth is critical in the formation of many types of ore deposits, for a variety of reasons. Look back through the deposit-type descriptions above and complete the following table describing which of those deposits types depend on a source of heat for their formation, and why.
| Deposit Type | Is heat a factor? | If so, what is the role of the heat? |
| Magmatic | ||
| Volanogenic massive sulphide | ||
| Unconformity-type uranium | ||
| Banded iron formation | ||
| Porphyry |
Exercise answers are provided Appendix 2.
Cobalt Deposits
Cobalt is an important component of lithium-based batteries, and the recent surge in the demand for batteries for electric vehicles and other purposes has resulted in increased demand for cobalt. Most of the world’s cobalt currently comes from sedimentary-rock hosted deposits in Zambia and Congo, Africa, although there is a significant amount that is mined along with nickel in magmatic and other deposits, especially those in Australia and Canada.
Media Attributions
- Figure 8.1.1 Steven Earle, CC BY 4.0, based on data current in 2020 from Visual Capitalist, https://www.visualcapitalist.com/
- Figure 8.1.2 Vale Nickel Mine by Timkal, 2008, via Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Vale_Nickel_Mine.JPG CC BY-SA 3.0
- Figure 8.1.3 (Left) Black Smoker by Butterfield, D. and Holden, J., National Oceanographic and Atmospheric Administration, public domain, http://oceanexplorer.noaa.gov/okeanos/explorations/10index/background/plumes/media/black_smoker.html; (Right) Diagram by Steven Earle, CC BY 4.0)
- Figure 8.1.4 Steven Earle, CC BY 4.0
- Figure 8.1.5 Panoramic view of Chuquicamata by Diego Delso, delso, CC-BY-SA 2.0, via Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Mina_de_Chuquicamata,_Calama,_Chile,_2016-02-01,_DD_110-112_PAN.JPG
- Figure 8.1.6 Black-band Ironstone by André Karwath, Museum of Mineralogy and Geology, Dresden, Germany, via Wikimedia Commons, CC BY-SA 2.5, via Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Black-band_ironstone_(aka).jpg
- Figure 8.1.7 Steven Earle, CC BY 4.0
- There is no doubt that mining companies will be attracted to jurisdictions where relaxed labour and environmental rules make it easier for them to make a profit. This is the nature of capitalism, and the onus falls upon the jurisdiction to make laws that will protect their environment and their workers, and on the mining companies to avoid exploiting workers and damaging the environment. ↵
- Galley, A., Hannington, M., & Jonasson, I. (2007). Volcanogenic massive sulphide deposits. In Goodfellow, W. (Ed.), Mineral deposits of Canada: A synthesis of major deposit-types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods. Geological Association of Canada, Mineral Deposits Division, Special Publication No. 5, 141-161. https://www.researchgate.net/publication/288005450_Volcanogenic_massive_sulphide_deposits_in_mineral_deposits_of_Canada_A_synthesis_of_major_deposit_types ↵
