2.5 Geosphere Earth Systems
Steve Earle
Most Earth system processes involve components of the geosphere and some critically important ones take place entirely within the geosphere. Most of these are not visible to us, and we only know about them because we can observe their effects when rocks and magma get brought to surface. We can then use chemistry and physics to understand how they happened.
One such process takes place within the crust on either side of a divergent boundary. Due to the heat of volcanism close to the boundary itself, water that is present in the cracks and pores of the oceanic crust gets heated and rises towards surface. This draws more ocean water into the crust from adjacent areas and a strong convection system develops that can continue for millions of years (Figure 2.5.1). One result is that the oceanic crust becomes metamorphosed through a process of hydrothermal alteration. There are several different metamorphic reactions that take place there. For example, at around 400⁰ C olivine (a magnesium or iron silicate) in the basalt reacts with water to create serpentine (a hydrated magnesium sheet-silicate mineral) plus brucite (a magnesium hydroxide mineral), as shown here:[1]
[latex]\ce{2Mg2SiO4 + 3H2O -> Mg3Si2O5(OH)4 + Mg(OH)2}[/latex]
olivine + water [latex]\ce{->}[/latex] serpentine + brucite
As a result of reactions like this one, much of the ocean crust—everywhere—is rich in minerals like serpentine and brucite that include the water in their structures.
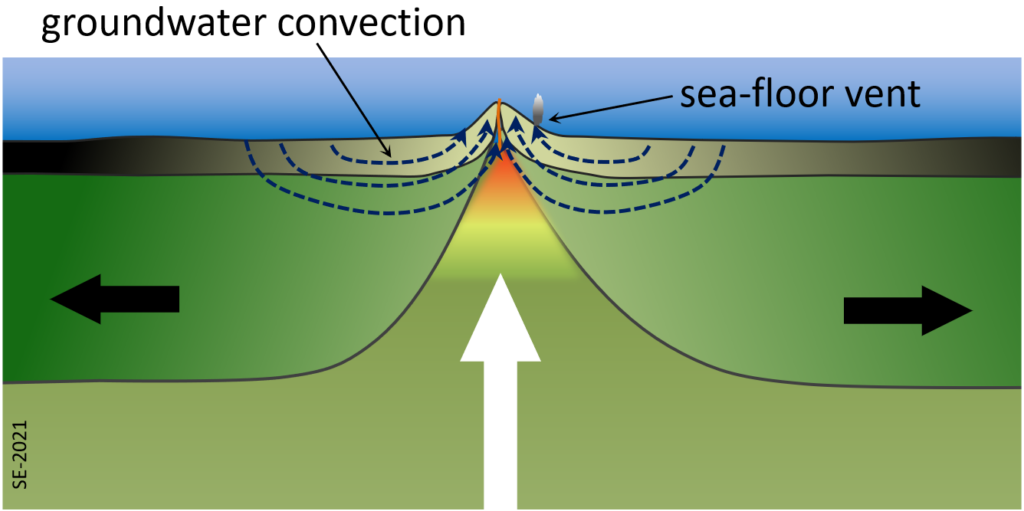
Wherever that hot groundwater flows out onto the seafloor it creates some interesting conditions. The groundwater in this setting tends to be quite rich in sulphur, along with various metals and when the hot groundwater meets the cold ocean the sulphur (as hydrogen sulphide) quickly reacts with metals to form sulphide minerals, such as pyrite:
[latex]\ce{2H2S + Fe^2+ -> FeS2 + 4H^+}[/latex]
The plume of tiny crystals of pyrite appear black and the result is known as a black smoker (Figure 2.5.2). In some cases, the sulphur is present in an oxidized form (as sulphate ions) and that combines with calcium ions to create the calcium sulphate mineral anhydrite.
[latex]\ce{Ca^2+ + SO4^2- -> CaSO4}[/latex]
In this situation the plume is likely to be white, so these are called white smokers.
Black or white, sea-floor hot spring vents are attractive places for sea life. Various microorganisms get energy from the unique chemical conditions, forming microbial mats around the vents. These are eaten by shelled organisms and crustaceans, which in turn provide food for larger organisms. All of this happens in complete darkness, making this one of the few ecosystems that doesn’t depend on sunlight as an energy source. Some have speculated that sea-floor vents are possible candidates for the origin of life on Earth.[2]

Another important geosphere Earth-system process takes place following subduction of oceanic lithosphere. This results in rock, sediment, water and biological matter that was present on the sea floor and part of the oceanic lithosphere being forced down into the mantle. When this material gets heated some of the water—including that within minerals like serpentine and brucite—gets released and comes back towards the surface above the subduction zone, but most of it, and everything else, continues down into the middle and lower mantle, where it eventually mixes with the rest of the mantle rock. Some of that material eventually comes back to surface via convection and magmatism, and if we look carefully at the chemistry of rocks produced from that magma it can be possible to see evidence of their long past history on the Earth’s surface.[3]
Media Attributions
- Figure 2.5.1 Steven Earle, CC BY 4.0
- Figure 2.5.2 Public Domain image by Normark, W. & Foster, D., East Pacific Rise, 21 degrees north. Base of “black smoker” chimney, Pacific Ocean. via Wikipedia, https://en.wikipedia.org/wiki/Hydrothermal_vent#/media/File:BlackSmoker.jpg
- Iyer, K. (2007). Mechanisms of serpentization and some geochemical effects [Doctoral thesis, Faculty of Mathematics and Natural Sciences, University of Oslo]. Duo Research Archive. http://urn.nb.no/URN:NBN:no-18920 ↵
- Martin, W., & Russell, M. J. (2007). On the origin of biochemistry at an alkaline hydrothermal vent. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 362(1486), 1887–1925. https://doi.org/10.1098/rstb.2006.1881 ↵
- Delavault, H., Chauvel, C., Thomassot, E., Devey, C., Dazas, B., (2016). Relics of Archean sediments in the Pitcairn plume. Proceedings of the National Academy of Sciences, 113(46), 12952-12956. https://doi.org/10.1073/pnas.1523805113 ↵
