14.5 Liquid Wastes
Steve Earle
North Americans produce approximately 250 litres of wastewater (a.k.a. sewage) per person per day. The actual amount varies from place to place, of course, but it is generally higher than in European countries (by several times in some cases), and much higher than in most other parts of the world. That liquid waste comes from toilets, showers and baths, laundry, and kitchens. Because North Americans use so much water, our wastewater is relatively dilute, with dissolved and suspended solids content in the range of 1000 to 2000 mg/L. In other words, each of us sends 250 to 500 g (dry weight) of solid matter down the sewer each day.
The solids in our waste include feces, food particles, toilet paper, grease, oil, soaps, dissolved salts and metals, and mineral matter (sand, clay etc.). Those components give wastewater chemical characteristics similar to those summarized in Table 14.5.1.[1]
| BOD5 | COD | Organic Carbon | Total Suspended Solids | Volatile Suspended Solids | Carbonates | N | P | Fats, Oils & Grease |
| 305 | 740 | 250 | 450 | 320 | 37 | 80 | 23 | 100 |
- Bacteria (including: Escherichia, Salmonella, Shigella, Campylobacter and Vibrio cholerae)
- Viruses (including: hepatitis, rotavirus, coronavirus, enterovirus)
- Protozoa (including: Entamoeba, Giardia and Cryptosporidium)
- Parasitic worms and their eggs
Most of the microorganisms in sewage are harmless to us, but some can lead to illnesses if wastewater is not treated or dealt with adequately. Unfortunately, inadequate treatment is common in most parts of the world. The problem is especially serious if wastewater mixes with drinking water supplies.
According to Erik Peterson, Resources Analyst at the World Health Organization, more than 1.5 million people (including over 400,000 children) died in 2019 as a result of diseases contracted from water, and “at any given time, close to half the population of the developing world is suffering from waterborne diseases associated with inadequate provision of water and sanitation service.”[3]
Pathogens from wastewater are a health problem. On the other hand, some other components of wastewater, including oxygen-demand and trace elements, are geological problems because they impact the quality and physical properties of water bodies, and aquatic ecosystems. Because of its high levels of nitrogen and phosphorous, wastewater can promote significant algal growth, which has undesirable aesthetic and ecosystem implications. In surface water some of the suspended components will gradually settle, but the smaller particles and the microorganisms will likely stay in suspension, and dissolved components will tend to stay in solution. Such contaminants can travel a significant distance.
Wastewater that is dispersed into an aquifer represents a very different problem than that which gets into a stream, or a lake or the ocean. In an aquifer the wastewater will interact with the minerals that surround it. Dissolved components will tend to become attached to surfaces, especially clay mineral surfaces, and so they may not be dispersed for more than tens to hundreds of metres. In general, aquifer materials act like filters, so suspended contaminants (including microorganisms) won’t get very far: typically metres to tens of metres depending on the nature and grain size of the aquifer. Because the supply of oxygen is limited at depth, there will be minimal oxidation of the organic constituents of the waste.
Organic matter and other components that make up the COD and BOD will consume oxygen from the water, and that will have negative implications for aquatic life. A condition called eutrophication can also arise from excessive nitrogen and phosphorus (from sewage) entering a body of water. Nitrogen and phosphorus are used in fertilizer for a very good reason: they occur naturally in low concentrations, and that restricts plant growth. When nitrogen and phosphorus are added to surface water, they contribute to rapid plant growth and algae blooms. Excessive algae can clog fish gills and block sunlight, resulting in dead organic matter. Compounding this problem, the algae will also eventually die and then it too becomes oxygen-demanding waste.
Wastewater Treatment
- The main goal of primary treatment is to separate the solids from liquids, and that is achieved first using screens (with openings of around 1 cm) to remove larger particles and then settling tanks to separate the smaller suspended material. Any material that floats is removed from the surface. The sludge that settles to the bottom is separated and can be further processed by fermentation or digestion with bacteria. Methane is produced during this process, and that can be used as fuel. Primary treatment typically removes about half of the BOD and the fecal coliforms and most of the suspended solids.
- During secondary treatment the wastewater is aerated and differing bacteria are added at different stages to break down the organic matter. About 85 to 90% of the BOD and suspended solids, along 90 to 99% of the coliform bacteria are removed at this stage. The water may then be filtered and disinfected with chlorine, ozone or UV light before being released to the environment.
- Tertiary treatment involves bioreactors with different levels of oxygenation and bacteria, or with specific chemical processes to remove dissolved components, especially phosphorous and nitrogen so that the released water doesn’t contribute to algal growth. This end-stage water may also be filtered and disinfected with chlorine, ozone or UV before being released to the environment.
An example of a wastewater treatment plant is illustrated on Figure 14.5.1. The round structures are aeration and settling tanks.

Most residents of rural areas are not connected to sewage collection piping networks and so are required to deal with their own wastewater (or else have it taken to a treatment plant in a tanker). The most common solution is a septic tank with a drainage field. The septic tank is typically a plastic or concrete tank with two chambers that is designed so that most of the solids can settle and the scum that develops on top of the wastewater is constrained. The liquid is allowed to flow to a drainage field similar to the one shown on Figure 14.5.2. The key features to note are that the plastic pipes have perforations to allow the liquid to drain out, and that a layer of permeable gravel has been placed in order to allow the liquid to drain slowly and enter the underlying soil or permeable rock. The premise behind an installation of this type is that as the water seeps slowly through the drainage medium and then the underlying natural soil and/or rock it will become sufficiently clean so that it won’t seriously contaminate the nearest body of surface water. For that reason, it is important to assess permeability and porosity of the natural materials present in the area where a drainage field is to be constructed. If the permeability is too high the wastewater may flow through too quickly to be effectively treated. If the permeability is too low the wastewater may not flow away at all, and instead will pool on the surface.
Exercise 14.4 What Happens to Your Wastewater?

It’s good practice to be aware of what happens to our waste—both solid and liquid!
If you live in a town or city your wastewater likely flows through a system of pipes to a treatment plant, where it is treated and then released to the environment. Find out where your wastewater goes, how it is treated (is it primary, secondary or tertiary?), what is done with the treated water, and what is done with the remaining solids (sludge). This type of information should be available on your city or regional service provider’s website. In some jurisdictions you might even be able to go on a tour of your wastewater treatment facility.
If you live in a rural area, you may have your own wastewater treatment system (e.g., a septic tank and drainage field). See what you can find out about that, and where the water eventually ends up.
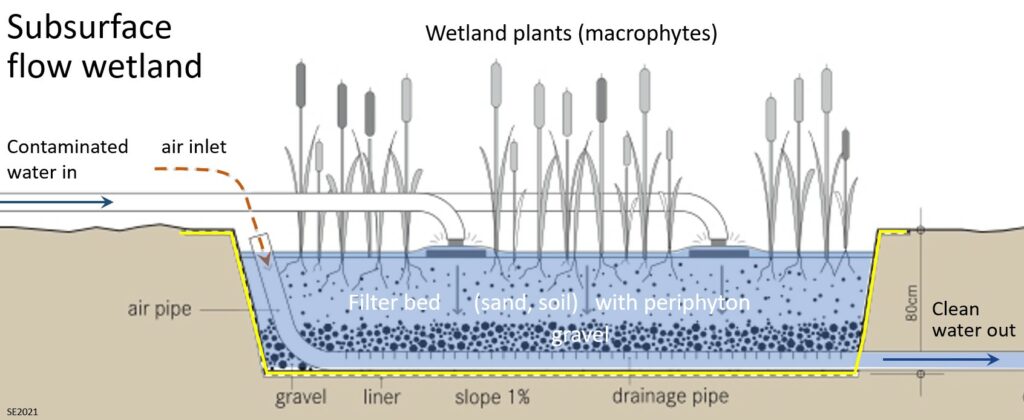
Before it is released to the natural environment effluent from a wastewater treatment plant, runoff from a road, or “grey water” from a kitchen or shower can be further decontaminated in a wetland. A constructed wetland is situated within a natural or excavated basin that has an impermeable liner. It has a perforated plumbing system at the base embedded in permeable gravel which is covered with finer material (Figure 14.5.3). Wetland plants (a.k.a. macrophytes) are grown within the wetland, but their primary function is to provide a substrate (roots, stems and leaves) and appropriate chemical conditions for a population of microorganisms (algae, bacteria etc.) called a periphyton. The periphyton is responsible for removal of about 90% of the pollutants. Well-designed constructed wetlands are effective in removing nitrogen, phosphorous and trace metals and in reducing BOD and COD levels.
Media Attributions
- Figure 14.5.1 Marlborough East Wastewater Treatment Plant by Nick Allen, 2015, CC BY SA 4.0, via Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Marlborough_East_Wastewater_Treatment_Plant_Aerial.JPG
- Figure 14.5.2 Drain Field in Progress by Nonztp, 2019, CC BY SA 4.0, via Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Drain_field_in_progress.jpg
- Figure 14.5.3 NZ0963: Waste water treatment works and transport depot, Low Prudhoe by Andrew Curtis, CC BY-SA-2.0, via Geograph, https://www.geograph.org.uk/photo/2039001
- Figure 14.5.4 Steven Earle, CC BY 4.0, after Tilley, E, et al. (2014). Compendium of sanitation systems and technologies (2nd edition). Swiss Federal Institute of Aquatic Science and Technology, via Wikimedia Commons, https://commons.wikimedia.org/wiki/File:Tilley_et_al_2014_Schematic_of_the_Vertical_Flow_Constructed_Wetland.jpg
- From the Technische Universitat Hamburg (accessed May 2021), https://cgi.tu-harburg.de/~awwweb/wbt/emwater/lessons/lesson_a1/lm_pg_1066.html ↵
- From: Wikipedia contributors. (2021, November 22). Sewage. In Wikipedia (accessed May 2021). https://en.wikipedia.org/wiki/Sewage ↵
- Berman, J. (2009, October 29). WHO: Waterborne disease is world's leading killer (accessed May 2021). Center for Strategic and International Studies. https://www.voanews.com/archive/who-waterborne-disease-worlds-leading-killer ↵
- Safe Drinking Water Foundation. (n.d.). Wastewater treatment: Wastewater treatment fact sheet. https://www.safewater.org/fact-sheets-1/2017/1/23/wastewater-treatment (Accessed May 2021) ↵
- Pescod, M.B. (1992). Wastewater treatment and use in agriculture - FAO irrigation and drainage paper 47. Food and Agriculture Organization. http://www.fao.org/3/t0551e/t0551e00.htm#Contents (Accessed May 2021) ↵

