10.5 Clay Minerals
Steve Earle
In Earth science the word “clay” has two meanings. Clay is broadly defined as any unconsolidated material with a grain diameter less than 0.004 mm. That is about 1/100th as big as the period at the end of this sentence, so is not big enough to see with the naked eye. This could include finely ground up quartz, feldspar, calcite, hematite or any other mineral. “Clay” also refers to the clay minerals, which are sheet silicates or phyllosilicates (from the Greek word phyllo meaning leaf). Clay minerals typically only exist as very tiny crystals, so most true clays also conform to the fine-grained meaning of the word “clay”.
A clay mineral is a finely crystalline sheet silicate with hydroxyl ions, and in some cases with water as part of the structure. By sheet silicate we mean that the silica tetrahedra are arranged in flat sheets, with strong covalent bonding within the sheets, and that these sheets are arranged in stacks, where the bonding between the sheets is relatively weak. A hydroxyl ion is an oxygen-hydrogen pair (OH–), and these form a part of all clay minerals. Some clay minerals also have H2O as part of the structure, or in some cases water is simply attached onto the structure.
Most of the clay present in rocks at surface has formed as a result of weathering of other silicate minerals, primarily feldspars, micas, pyroxene and amphibole. The reactions involved are hydrolysis reactions, something like the following reaction of potassium feldspar plus water and carbon dioxide to kaolin.[1]
2KAlSi3O8 + 2CO2 + 11H2O —> Al2Si2O5(OH)4 + 2K+ + 4H4SiO4 + 2HCO3–
K-feldspar + carbon dioxide + water -> kaolin + potassium, silica and bicarbonate ions in solution
In simple terms, K-feldspar reacts with water and carbon dioxide to form kaolin plus bicarbonate ions. The potassium and some of the silicon that were originally present in the feldspar, are removed in solution. The CO2 comes from the atmosphere, and over geological time, this is type of reaction plays an important role in controlling the atmosphere’s composition and hence the greenhouse effect.
Clay minerals can also be formed when hot waters (known as hydrothermal solutions) circulate through a body of rock. As is the case for weathering, the hot solutions lead to alteration of pre-existing minerals. Hydrothermal solutions are often also associated with the formation of metal deposits (such as porphyry copper deposits) and the surrounding clay-mineral halos can be an important guide in the exploration for such deposits.
Unlike the primary silicate minerals that they form from, clay minerals are soft and easily eroded into tiny fragments and then transported. They accumulate mostly as sediments in low-energy deposition environments (e.g., deep ocean or in lakes), sediments that are eventually turned into shale.
Clay Mineral Structures
Clay minerals are comprised of silica tetrahedra and alumina octahedra, which are illustrated on Figure 10.5.1. As we’ve seen in Chapter 2 (Figure 2.1.5), a silica tetrahedron is a silicon ion surrounded by four oxygen ions. Planes drawn through lines connecting the oxygens atoms define a tetrahedral (four-surfaced) shape. An alumina octahedron is an aluminum ion surrounded by six oxygen or hydroxyl ions. Planes drawn through lines connecting the oxygens and hydroxyl atoms define an octahedral (eight-surfaced) shape.
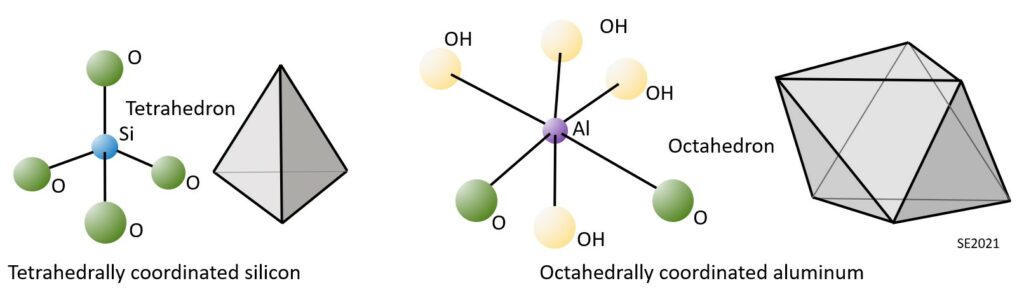
An important feature of clay minerals results from the characteristics of their bonding. The tetrahedra and octahedra are strongly bonded to each other within the sheets, but the sheets are only weakly bonded one to another. The sheets that make up a clay mineral grain have a tendency to slide with respect to each other, and the result is that clay mineral masses tend to be soft and plastic, and not very strong.
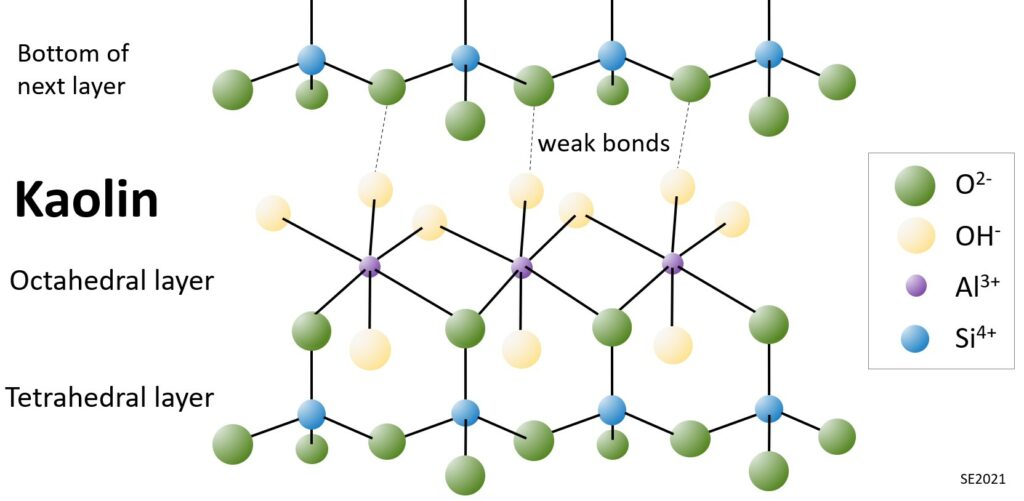
The simplest clay mineral is kaolin. Each “sheet” within the kaolin structure is comprised of a silica tetrahedral layer and an aluminum octahedral layer (Figure 10.5.2). The combination of one tetrahedral layer and one octahedral layer makes this a 1:1 layer silicate. For simplicity, it may be useful to describe this as a T-O structure (1 tetrahedral layer and 1 octahedral layer). This structure is also found in the mineral serpentine, in which magnesium substitutes for aluminum in the octahedral sites.
The structure of illite is more complicated than that of kaolin. In this case each “sheet” within the structure is comprised of an aluminum octahedral layer sandwiched between two tetrahedral layers (one “right side up” and the other “up-side down”) (Figure 10.5.3). Illite also has potassium ions situated at specific sites between the sheets. The combination of two tetrahedral layers surrounding one octahedral layer is known as a 2:1 layer silicate. We can also describe this as a T-O-T structure. Some other T-O-T clays include smectite, talc and chlorite.
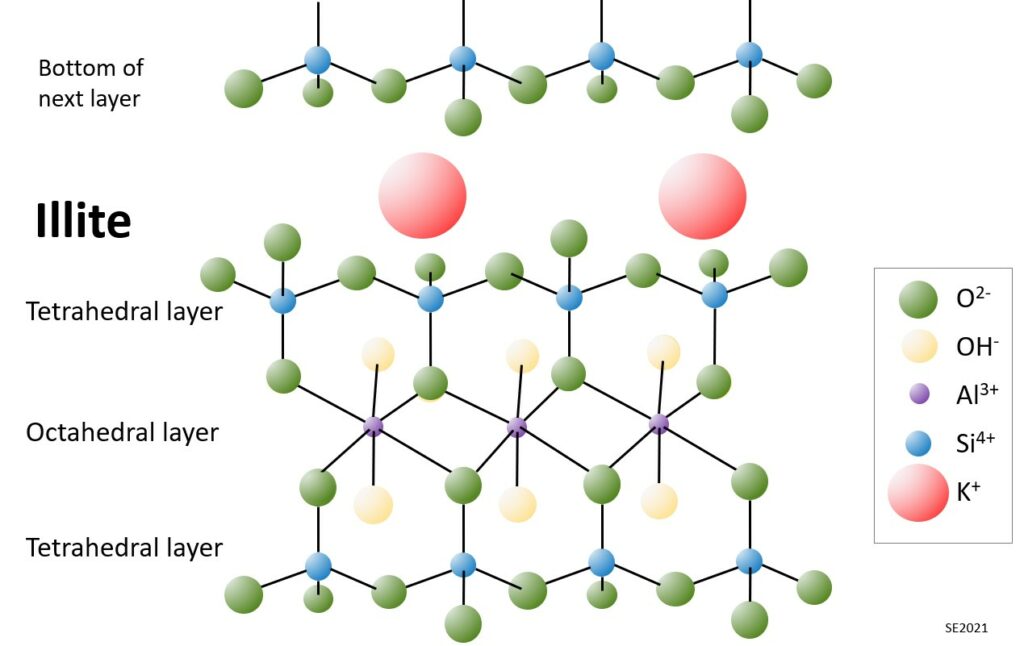
The potassium cations (K+) of illite are held in place because the upper and lower surface of each layer is saturated with oxygen ions (O-2) giving these surfaces a consistent negative charge. These negatively charged surfaces are one of the fundamentally important features of clay minerals, because such surfaces are attractive to positively charged ions, such as heavy metals, or some organic pollutants. Not only do clays have these attractive surfaces, but they have very large surface areas. It is estimated that a cubic centimetre of clay has a reactive surface of around 2800 square metres, which is equivalent to the area of a football field!

Some kaolin crystals are shown Figure 10.5.4. The kaolin plates are stacked up in loosely defined strands. Each visible plate is between 1/10th and 1/5th of a micron thick, but each of these is made up of hundreds to thousands of the T-O layers illustrated in Figure 10.5.2. There is a significant amount of empty space between the plates.
Although there are many dozens of different clay minerals, there are just a handful that are important for us to be aware of here, and these are summarized in Table 10.3. The first thing to note is that there are only two 1:1 (T-O) clay minerals in this list, kaolin and serpentine, while all of the others are 2:1 clays (T-O-T). Most of these 2:1 clays have magnesium and/or iron in them, and so can be considered ferromagnesian silicates. The only non-ferromagnesian clays listed are kaolin, pyrophyllite and Illite, although Illite can also have small amounts of magnesium, and the glauconite variety has iron. The important point here is that while kaolin, pyrophyllite and Illite are typically derived from alteration of minerals like feldspar and muscovite, most of the other clays are derived from alteration of minerals like olivine, pyroxene, amphibole and biotite.
| Clay Mineral | Type | Typical Chemical Formula | Variations and (other names) |
| Kaolin | 1:1 | Al2Si2O5(OH)4 | kaolinite, dickite, halloysite, nacrite |
| Serpentine | 1:1 | Mg3Si2O5(OH)4 | antigorite, chrysotile (asbestos), lizardite |
| Illite | 2:1 | K0.65Al2.0(Al0.65Si3.35O10)(OH)2 | glauconite, (hydromuscovite, K-deficient muscovite) |
| Pyrophyllite | 2:1 | Al2Si4O10(OH)2 | |
| Smectite | 2:1 | (Na, Ca)0.33(Al, Mg)2(Si4O10)(OH)2 ∙ nH2O | montmorillonite (bentonite), saponite, nontronite |
| Vermiculite | 2:1 | (Mg, Fe2+, Fe3+)3 ((Al, Si)4O10)(OH)2 ∙ 4H2O | |
| Talc | 2:1 | Mg3Si4O10(OH)2 | |
| Chlorite | 2:1 | (Mg, Fe)3(SI, Al)4O10(OH)2 ∙ (Mg, Fe)3(OH)6 | clinochlore, pennantite, chamosite, sudoite |
Formation of Clay Minerals
As already noted, clay minerals typically form from the alteration (hydrolysis) of pre-existing silicate minerals. The type of clay mineral that will form in any situation depends partly on what silicate mineral is being altered, but also on a range of other variables such as the temperature and pressure, and the chemistry of the solutions that are passing through or over the rock at the time.
Clay minerals form during weathering at surface, during hydrothermal alteration of rock within the crust, and during the diagenesis (mineral alterations that take place when sediments get buried beneath other sediments) and their transformation into sedimentary rock.
While the temperatures and pressures of hydrothermal alteration and diagenesis can vary dramatically, weathering conditions are broadly similar the world over, the main differences being the amount water available from precipitation and the average temperatures. Temperature differences of a few tens of degrees mainly control the rate of weathering, not the type, and so the major factor that determines which clay minerals will form during weathering is the type of primary silicate minerals present in the rock.
A summary of the clay products of weathering of primary silicate minerals is provided in Table 10.4. Quartz in not in this list because it isn’t subject to chemical weathering.
| Primary Silicates | Typical Clay Minerals That Will Form Under Weathering Conditions |
| Olivine | smectite |
| Amphibole & pyroxene | smectite, talc, vermiculite & chlorite |
| Plagioclase feldspar | kaolin (especially halloysite or kaolinite) |
| Potassium feldspar | kaolin (and illite less commonly) |
| Biotite | vermiculite, kaolin |
| Muscovite | Tends to be generally resistant to weathering but can convert to illite |
The clay mineral products listed Table 10.4 are specifically those that form under weathering conditions, which generally means temperatures under 40° C, atmospheric pressure, and water that has low levels of dissolved ions and close to neutral pH.
The higher temperatures associated with burial and diagenesis of sediments, or with hydrothermal alteration or even low grade metamorphism, result in the formation of some clays that are not typically produced during weathering.
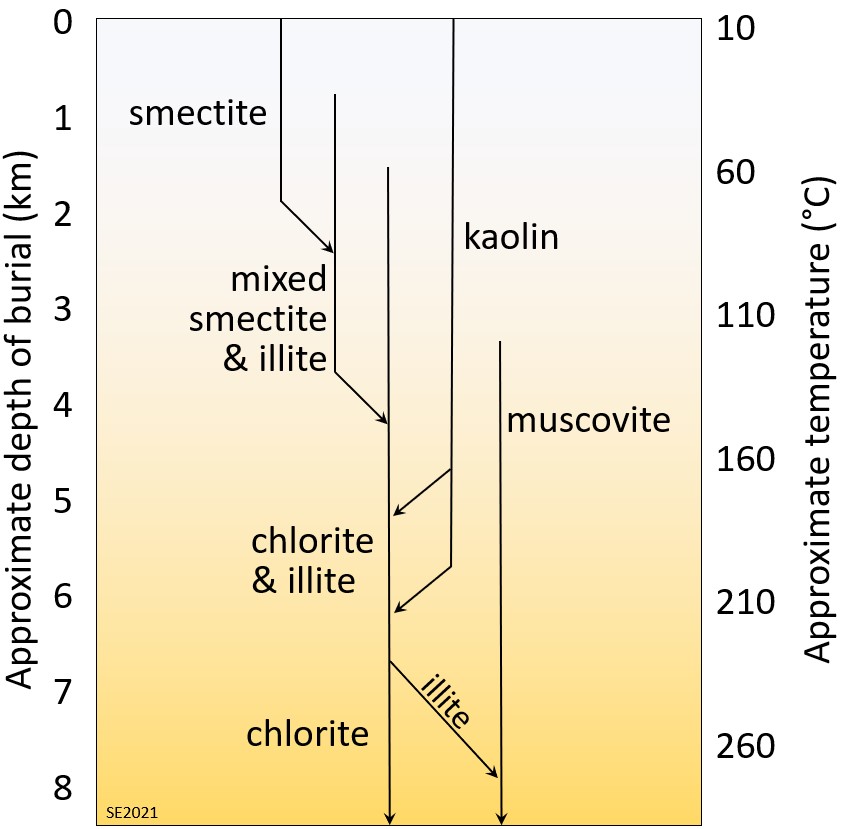
Some of the clay-mineral transformations that can take place within sediments as they undergo progressively greater burial beneath other sediments are illustrated on Figure 10.5.5. The assumption here is that the sediments already include some clay minerals, especially the low-temperature clays smectite and kaolin produced during weathering in the sediment source area. Starting at around 100° C, the smectite might first be altered to a mineral with mixed or alternating layers of smectite and illite. The mixed-layer clays may be altered to chlorite and illite at around 150° C, with the illite being altered to muscovite at over 200° C. Any kaolin originally present in the sediment as kaolinite or halloysite might first get transformed to the higher temperature polymorphs (dickite or nacrite) and then to illite, and eventually to either chlorite or muscovite.
Hydrothermal alteration takes place where hot water circulates at depths of hundreds to thousands of metres within the crust. This is commonly associated with convection systems produced by magmatic heat, and as described in Chapter 8, it is a process that is commonly associated with ore-formation. Clay alteration is well known around porphyry, epithermal, volcanogenic massive sulphide and some uranium deposits. In these environments water chemistry can be extremely variable, and temperatures can range up to hundreds of degrees, so a very wide range of clay minerals and other alteration minerals can form. The details are beyond the scope of this book but can be found in works related to mineral deposits.
Hydrothermal alteration to clay minerals also takes place in volcanic areas because the heat source can drive convection and will also speed up the reaction rates. This is evident around Mount Meager area in BC’s Coast Range (Figure 10.5.6). Some of the rock colours are a product of clay alteration. The scar from the 2010 rock slide and rock avalanche—which happened in part because the volcanic rock had been weakened by hydrothermal clay-alteration—is visible in the photo.

One of the most important sites of clay alteration is at oceanic divergent boundaries where there is volcanic heat resulting in convective water circulation. Oceanic crustal rocks include basalt and gabbro and even ultramafic rock at greater depth, so they are rich in pyroxene and olivine, and these minerals are readily altered to minerals like chlorite and serpentine at temperatures of a few hundred degrees. An example of that process is as follows:
2Mg2SiO4 + 3H2O → Mg3Si2O5(OH)4 + Mg(OH)2
(olivine + water -> serpentine + brucite (not a clay mineral)
The resulting rock may look like that shown in Figure 10.5.7. The pyroxene and olivine of oceanic crust may also be altered to smectite (at low temperatures) and to talc and chlorite. A significant proportion of oceanic crust has been altered in this way, and since oceanic crust makes up about 70% of the Earth’s crust, this is likely the largest repository of clay minerals on the planet. That is significant from an Earth-systems perspective because when oceanic crust is eventually subducted the clay minerals are heated and converted (by metamorphism) back to non-hydrous silicate minerals and the water is released. This water contributes to partial melting above a subduction zone, and therefore to composite volcanoes along subduction boundaries.
Exercise 10.5 Clay Mineral Origins
Some clay minerals are listed below. Indicate the environment (e.g., weathering, diagenesis, hydrothermal alteration) in which it likely formed, and a possible precursor mineral.
| Mineral | Likely Environment of Formation and Possible Precursor |
| Halloysite | |
| Mixed-layer clay | |
| Serpentine | |
| Illite | |
| Dickite |
Exercise answers are provided Appendix 2.
Properties of Clay Minerals
It is worthwhile to understand some of the properties of clay minerals, as they have important implications for many aspects of environmental geology. They play a role in a great variety of processes, from soil chemistry to the causes and effects of earthquakes, to the permeability of rocks. Some of their important properties are as follows:
They are soft and weak, primarily because of the weak bonds between sheets and the resulting tendency for the sheets to slide past each other under stress. Talc is number 1 on the Mohs scale, and most other clay minerals are similarly soft. The weakness of clay minerals has implications for slope failure (as noted above) because clay bearing rocks also tend to be weak, and also for earthquakes, because a plate boundary with clay-rich rocks is likely to slide smoothly, and so less likely to stick and cause large earthquakes.
Most clays are malleable when wet—also because of weak inter-layer bonds—and so can easily be formed into useful shapes for artistic, domestic, industrial and scientific purposes.
Clay minerals are crystals like other minerals, but they typically only form as very small crystals, so clay deposits are almost universally fine grained. Although a body of clay has significant porosity, the pores are extremely small and most of the water within them is close enough to a grain boundary to be held tightly by surface tension, making a clay deposit significantly impermeable. This has implications for groundwater flow (Chapter 11) and for waste disposal (Chapter 13).
The tetrahedra and octahedra that make up clay minerals have negatively charged ions (anions) on their outsides (either O2- or OH–) making the surfaces of the individual layers negatively charged, and therefore attractive to positively charged ions (cations) in solution. Most metals exist as cations and many organic pollutants have positive charges, and so clay minerals are efficient scavengers of environmental pollutants, and can be used as barriers to prevent dispersal of contaminants and also in environmental rehabilitation projects. Different clays have different capacities to absorb cations (known as “cation exchange capacity”), and some of these are listed in Table 10.5.[2] Smectite has a much higher cation exchange capacity than other clays because cations can get onto the sites in between the molecular layers within a crystal, as opposed to just the outside surfaces of the crystals.
| – | Effective Surface Area in m2/g | – | Cation Exchange Capacity |
| Mineral | Interlayer | External | Meq/g* |
| Kaolin | 0 | 15 | 1 to 10 |
| Chlorite | 0 | 15 | <10 |
| Illite | 5 | 15 | 10 to 40 |
| Smectite | 750 | 50 | 80 to 150 |
The smectite clays have a 2:1 structure similar to that of illite (Figure 10.4.3) but the interlayer cations are typically either sodium, calcium or magnesium (instead of potassium). This means that the layers are a little further apart than in illite, and for that reason smectites have a unique ability to absorb water molecules in the interlayer sites. This is especially true for sodium smectites, which can absorb up to 18 layers of water in between the sheets, and thereby will expand or swell dramatically when wet. Some swelling clay is shown on Figure 10.5.8. The clay is present within a depression and was wet. On drying it shrank in the typical mudcrack pattern. Swelling clays have a number of important industrial and domestic uses, but they also have some serious geological implications. A swollen wet smectite is even weaker than a dry one, so can weaken slopes significantly, and bodies of swollen clay can distort the materials around them, also potentially contributing to slope failure or problems for building or road foundations.

Vermiculite will also swell slightly when wet, but, unlike other clays it will expand dramatically on heating (Figure 10.5.9). When heated to 500 to 800° C the water trapped between the layers will boil and push the layers apart increasing the volume dramatically. Expanded vermiculite has many uses, including as a growing medium, insulation, brake linings, and fireproof panels.

Exercise 10.6 Find Some Clay in Your Neighbourhood
Clay minerals are everywhere. Look around your neighbourhood or your region to see if you can find some. Likely places might include: a rock outcrop that is being weathered, a rock that has been hydrothermally altered, a fine-grained sedimentary rock (e.g., mudstone), a dried up puddle, the edge of swamp or pond, or a small bay. If you can’t find any place like those suggested, think about where there might be some clay that you can’t see such as in the middle of a lake or in the ocean.
You might also be able to find some clays at home. Look in the medicine cabinet for example, or amongst some arts and craft supplies.
And then there are likely to be things in your house made out of clay (or that were clay, and are no longer).
Clay Minerals and Earth Systems
- Clay minerals may have played a role in the initial evolution of life from organic chemicals because the regular structure of the clays could have acted as a template for the assembly of organic molecules,
- Conversion of silicate minerals to clay consumes atmospheric CO2 and so has climate implications,
- Clay minerals accumulate trace elements that later become available to plants and microorganisms,
- Clay minerals accumulate trace elements that may eventually get concentrated into mineral deposits,
- Clay minerals can reduce rock strength and so contribute to erosion and slope failure,
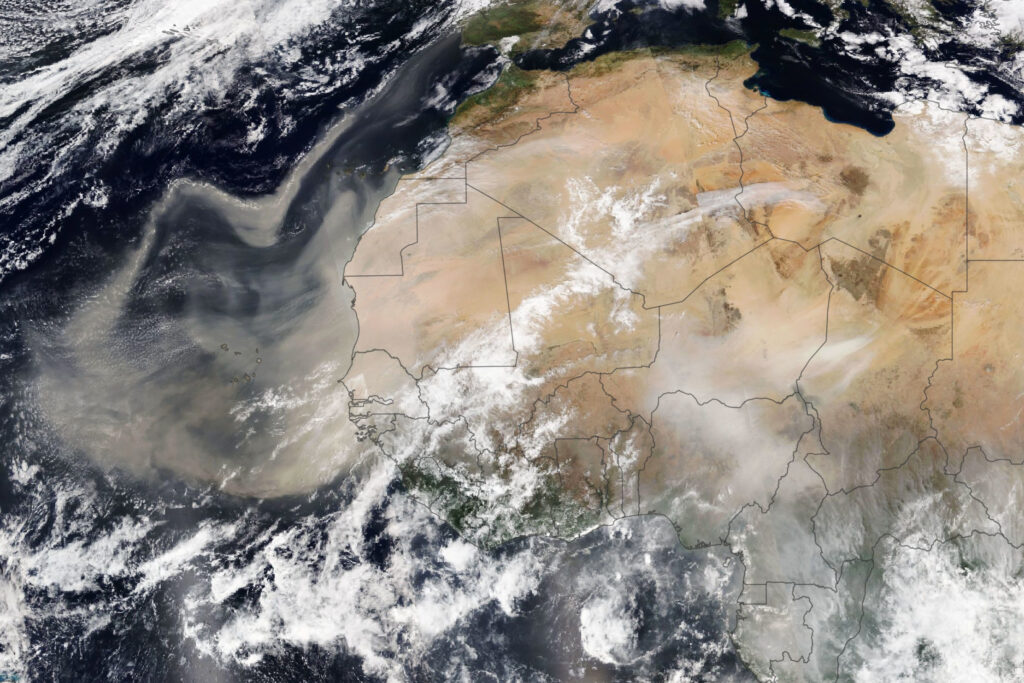
- Clay minerals suspended in water or as clouds of dust can be vehicles for the transfer of trace and major elements from land into the ocean (See Figure 10.5.10), and
- Clay minerals are the vehicles for the transfer of water from subducted oceanic crust into the mantle, leading to magma formation (via flux melting) and volcanism.
Media Attributions
- Figure 10.5.1 Steven Earle, CC BY 4.0
- Figure 10.5.2 Steven Earle, CC BY 4.0
- Figure 10.5.3 Steven Earle, CC BY 4.0
- Figure 10.5.4 Plates of the Clay Mineral Kaolin from ACEMAC Nano Scale Electron Microscopy and Analysis Facility, University of Aberdeen by GSoil, CC BY 3.0, https://www.abdn.ac.uk/business-info/facilities-and-expertise/acemac-nano-scale-electron-microscopy-and-analysis-facility-846.php#panel861
- Figure 10.5.5 Steven Earle, CC BY 4.0, after Figure 7.13. In Prothero, D. and Schwab, F. (2004). Sedimentary geology: An introduction to sedimentary rocks and stratigraphy (2nd ed.). Freeman and Co.
- Figure 10.5.6 Photo by Isaac Earle, CC BY 4.0
- Figure 10.5.7 Serpentinite by James St. John, CC BY 2.0, via Flickr, https://www.flickr.com/photos/jsjgeology/16940796272
- Figure 10.5.8 Photo by Steven Earle, CC BY 4.0
- Figure 10.5.9 Vermiculite by KENPEI, 2008, CC BY-SA 2.1 JP, via Wikimedia Commons https://commons.wikimedia.org/wiki/File:Vermiculite1.jpg
- Figure 10.5.10 Africa Dust from NASA, public domain, https://eoimages.gsfc.nasa.gov/images/imagerecords/147000/147952/africadust_virs_202149_lrg.jpg
- There are several different forms of the mineral kaolin. The best known is kaolinite. Others include halloysite, which forms at low-temperatures, and dickite and nacrite at higher temperatures. ↵
- The data in Table 10.5 are based on information in Wilson, M. (2004). Weathering of the primary rock-forming minerals, processes, products and rates. Clay Mineralogy, 39(3), 233-66. doi:10.1180/0009855043930133; and in Deer, W., Howie, R., and Zussman, J. (1991). An introduction to the rock-forming minerals (2nd ed). Longman. ↵

